| Molecular Probes
The Sutcliffe lab has chosen peptides as their molecular probes. The chemical advantages of peptides include ease of preparation, modification and incorporation of a number of radioisotope-containing ‘prosthetic’ groups. On the biological side, they are attractive targeting agents for molecular imaging because of their fast distribution in the body and rapid clearance from non-target tissues. The Sutcliffe lab uses two approaches to probe design, a ‘random’ and a ‘rational’ approach. In the random approach, peptide libraries on solid-phase beads are screened in cell based assays to identify new target-specific peptide sequences. The rational approach, by contrast, is based on structures found in natural ligands.
|
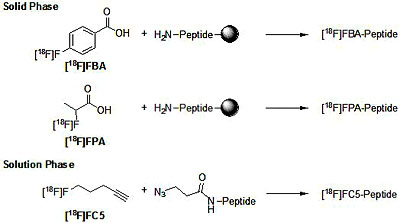 New Approaches to Labeling with 18F New Approaches to Labeling with 18F
The radioisotope fluorine-18 (18F) has a half-life of 109.8 minutes, thus requiring fast and reliable high-yield approaches to radiolabeling. Solid-phase radiolabeling is particularly attractive as it utilizes reliable peptide synthesis protocols for site-specific labeling and allows for easy removal of excess reagents by simple filtration. This approach, initially developed by the PI, was extended to include automated solid-phase peptide synthesis (SPPS) radiolabeling procedures using 4-[18F]fluorobenzoic acid and 2-[18F]fluoropropionic acid as prosthetic groups. The prosthetic group is selectively introduced to an N-terminal amino group or a deprotected lysine side chain.
In cases where solution-phase labeling is preferred (e.g. if cleavage of the peptide off the solid support could interfere with a functional group) site-specific labeling can be challenging. The Sutcliffe lab adapted the recently discovered CuI catalyzed 1,3-dipolar Huisgen cycloaddition (“Click” chemistry) for radiolabeling. Click chemistry relies on the selective cyclization of an azido-group with a terminal alkyne. Since this cycloaddtions is orthogonal to any other functional group it can be performed in the absence of protecting groups. Even though the Huisgen cycloadditions afford the product in excellent yield and purity, they usually require long reaction times or elevated temperatures to completion. By carefully choosing reaction conditions and catalysts reaction times were shortened to 10 minutes at room temperature, permitting rapid “click” radiolabeling with [18F]fluoro-compounds.
|
 High-Throughput Technologies High-Throughput Technologies
One-bead-one-compound (OBOC) molecular libraries have been extensively used in cell-bead binding assays to identify selective ligands against cell surface receptors, including cancer-related targets. OBOC peptide libraries provide a means of generating large numbers of potential ligands from which effective molecular targets can be identified. However, the selection of lead ligands from these random libraries remains a serious bottleneck. The Sutcliffe lab is developing approaches to streamline the lead compound search process both in vitro and in vivo.
|